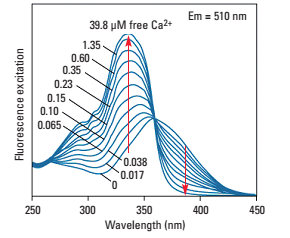
In vitro intracellular calcium imaging technology is to monitor the changes of intracellular calcium ion concentration by loading calcium indicator into the cell, using the characteristics of the fluorescence intensity or spectral properties of the calcium indicator being combined with calcium to monitor the changes in intracellular calcium ion concentration. At present, the commonly used calcium indicator is mainly the chemical fluorescent indicator Fura-2, which changes its spectral properties in the calcium-free and calcium-bound state. For example, in the calcium-bound state, its excitation peak changes from 363nm to 335nm, and the emission peak There is no obvious change, so dual wavelengths (340nm and 380nm) are usually used for two consecutive excitations, and the ratio of the emitted light intensity obtained after excitation is the relative value of the calcium signal.
Using research grade inverted fluorescence microscope, 340/380 LED fluorescent light source and high-sensitivity SCMOS camera, combined with perfusion and drug delivery system, it can finally perform real-time Fura-2 calcium imaging on Fura-2 AM-loaded in vitro cells and record calcium curve Variety.

Calcium imaging involves the use of calcium indicators, which are fluorescent molecules that respond to the binding of Ca2+ by producing fluorescence, which can be detected. Calcium indicators can either be chemicals that are added to the cell system, or cells can be genetically modified to produce their own (genetically encoded calcium indicators, or GECI).
The plethora of Ca2+ indicators that are available nowadays enable researchers to pick the optimal one for their purpose, considering the required Ca2+ sensitivity, fluorescent properties and speed. However, two points they all have in common are the need for speed (in terms of acquisition) and the requirement to use as little excitation light as possible to avoid bleaching and accompanying side-effects.
Compared with chemical dye calcium imaging, GECI imaging has more advantages in image dynamic range and signal-to-noise ratio. At the same time, it performs better in dynamics. It can even detect a single action potential, and it can also detect a large number of neuronal activities and neural subassemblies Cell (such as dendritic spines and axons) activity.
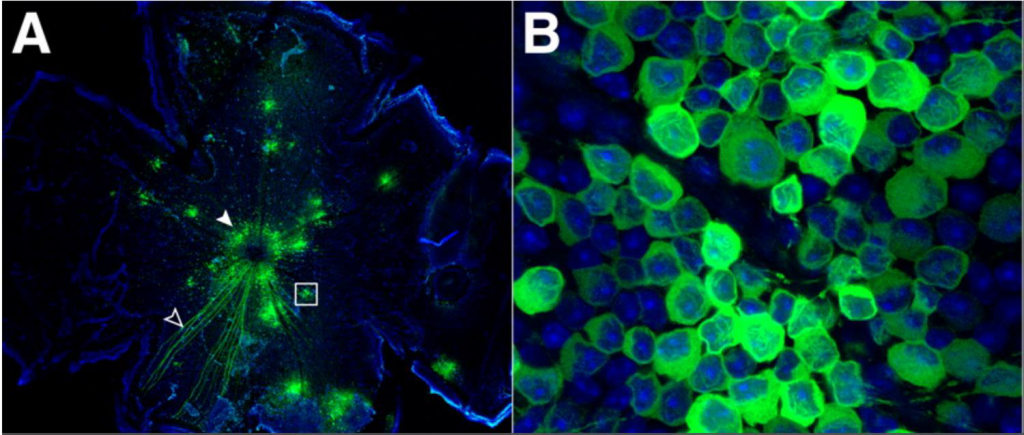
Calcium imaging with a confocal microscope. Image shows calcium indicator GCaMP3 (green) and nuclear stain DAPI (blue), imaging neurons from the eyes of an adult mouse. Image from Borghuis et al. (2011)
Applications range from imaging neurons and neuroendocrine cells to imaging Ca2 + sparks in cardiomyocytes. As the application changes, the required frame rate also changes: it may need to be between 0.1 Hz and 1 kHz, which places high demands on imaging cameras, requiring high-sensitivity, high-frame-rate scientific CMOS cameras.